How to calculate the pH of very weak acids and bases
Calculation for a Strong Acid
Let’s start with a classic school chemistry exercise to warm up:
If we have a 0.1 M solution of sulfuric acid, what is its pH?
Sulfuric acid (
Recall that the unit ‘M’ (“molar”) represents
The dissociation of sulfuric acid in water can be represented as:
So, our 0.1 moles of
The concentration of
The pH of the solution, using its definition, is
This is very acidic! It would turn universal indicator paper a deep red.
This calculation is straightforward. But what if we have a weak acid?
Calculation for a Weak Acid
Suppose instead we have a 0.1 M solution of acetic acid (
The dissociation of acetic acid in water can be represented as:
The equilibrium constant for this reaction is called the acid dissociation constant,
For acetic acid, it has been experimentally determined that
In any aqueous solution where acetic acid is present, the above relationship between those three species always holds true at equilibrium - no matter what else it is mixed with.
The usual method to solve for the pH of a weak acid solution is to set up an ICE table (initial, change, equilibrium), as follows:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| - | |||
| + | |||
| + |
Note that it doesn’t matter whether the units in our table are moles (amount) or molar (concentration), since the volume of the solution is constant. Here, we’ll use molarity, so we don’t need to worry about the volume.
Our solution starts with 0.1 M undissociated acetic acid, and no protons or acetate ions. During dissociation,
We now apply the equilibrium constant expression to the last column of the table:
With a little algebra, we can rearrange this into a quadratic equation:
We can solve this equation using the quadratic formula to get solutions of
Since
Now that we know that
This is over 100 times less acidic than our sulfuric acid solution despite having equal concentrations, so the distinction between strong and weak acids is very clear!
Calculation for a VERY Weak Acid
This time, suppose we’re looking at organic molecules that we usually wouldn’t think of as acids - even though they can be. For example, benzyl alcohol (
In comparison to phenol (
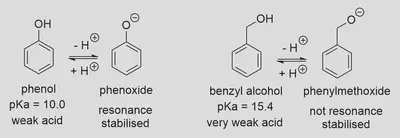
Let’s try to calculate the pH of a 0.1 M solution of benzyl alcohol. Intuitively, we should expect the answer to be very close to 7, since the acidity barely moves away from the pH of neutral water (7.0 at 25 °C).
First of all, let’s convert from
Therefore, the value of
Now, we’ll attempt to use the same method as before, with an ICE table:
| Species | Initial (M) | Change (M) | Equilibrium (M) |
|---|---|---|---|
| - | |||
| + | |||
| + |
Using the equilibrium constant expression on the last column, we get
Rearranging this into a quadratic equation gives us:
Solving for
So, we take the solution of
This is very strange! Why is the pH greater than 7, implying an alkaline solution?
This must be wrong. Let’s find out why and how to fix it.
The limitations of the ICE table method
When we set up our ICE table, we made the assumption that the initial value of
But, we should know that pure water experiences its own equilibrium reaction called autoionisation:
The equilibrium constant for this reaction is called the ionic product,
This means that in pure water, there is a constant concentration of
But in our ‘very weak acid’ example, the dissociation of benzyl alcohol is so weak that it becomes comparable to the autoionisation of water. In fact, the water becomes the dominant source of acidity in the solution.
The correct way for very weak acids
Rather than rely on the ICE table method, we’ll tackle this problem from first principles.
We can use the trick of setting the volume of the solution to 1 L, so that moles and molarity have the same numerical value, for convenience. So, we are adding 0.1 moles of benzyl alcohol to 1 L of water.
Once we add the benzyl alcohol, we will generate a solution containing four different ionic species:
as well as the neutral water itself.
We have two different equilibrium reactions happening in the solution:
- The autoionisation of water:
- The dissociation of benzyl alcohol:
We know equilibrium constants for both of these reactions, so we can write down two equations:
That’s two equations in four unknowns, so we can’t solve it just yet. We need to find two more constraints to help us out.
We know that the total amount of our organic compound can’t change, since it’s not present in the water originally. So, we can consider conservation of mass for our compound:
because we started with 0.1 moles of benzyl alcohol in 1 L of water.
Next, we know that the solution must be electrically neutral, since charges always balance out. So we can get a fourth equation from conservation of charge:
since the total positive charge present must be exactly balanced by the total negative charge present.
Now that we have four equations in four unknowns, we can solve them simultaneously to find our concentrations at equilibrium. If we do the algebra to eliminate all the variables other than
where
Cubic equations are tough to solve algebraically. We can solve it numerically instead to get three possible roots:
(all units in molar). As before, we take the positive solution of
Solving systems of nonlinear equations like that is a bit time consuming, and since we resorted to using a numerical solver, we could have just used one from the beginning. Using WolframAlpha to solve our system of four equations, and ignoring extraneous solutions, we find the appropriate solution to the system is:
so the value of
Finally, we can calculate the pH of the solution:
This is just barely below 7, which is exactly what we expected. The benzyl alcohol is such a weak acid that it has a negligible effect on the pH - but quantifying that effect precisely takes mathematical work!
Some technicalities
Aqueous protons
It’s worth noting that even though we’ve been writing
Using the ‘proper’ formula, the autoionisation of water would therefore be:
as one water molecule transfers a proton to another through a hydrogen bond. At no point is the proton ‘free’ in the usual sense.
Activity vs concentration
Throughout, we’ve been using concentration equilibrium constants,
Activity is simply a way to quantify the ’effective’ concentration of a species. For example, if some of the ions in solution get ‘stuck’ to the hydrophilic walls of the container, they are much less available for reaction, so we say that their activity is lower, dropping the overall effective concentration.
To calculate pH, we should really be using the activity of protons,
If all activity coefficients are equal to 1 (i.e. ideal behaviour, no species are ‘getting stuck’), then all we are doing is removing the units from our concentrations without changing the numerical value, so our answers are all the same. But for our very weak acid calculation, the concentrations involved are so tiny that the usually small nonidealities in activity could have a significant effect on the final answer, in the same way that neglecting the autoionisation of water did as shown above.
Temperature dependence of equilibria
Additionally, like all physical or chemical changes, there is some associated enthalpy change, meaning there will be some amount of heat released or absorbed during the process. For acid dissociations, this is usually an exothermic process, so heat is released, slightly warming the solution for some time. Since equilibrium constants are strongly temperature dependent, the ratios of the ion concentrations would change too, affecting the transient pH while the heat is dissipated. Due to the high heat capacity (’thermal inertia’) of water, this effect is likely to be small, and it would be would be compensated by a shift in the equilibria to lessen the exothermic dissociation reactions (by Le Chatelier’s principle), but it still may be worth considering for high precision work.
Another effect could be the thermal expansion (density reduction) of the solution on the slight heating, increasing the volume and diluting the concentrations, changing the equilibrium ratios again.
Volume of the solution
We have assumed that the volume of the water does not change when we add the benzyl alcohol. But at room temperature, pure benzyl alcohol (and acetic acid) are both liquids, so they occupy some finite volume too. But there is no guarantee that volume is conserved when we mix: for example, when we add sodium chloride (
I wasn’t able to find literature values for the excess volume of benzyl alcohol in water (with the possible exception of this paper that I can’t access!). The total volume of the solution is likely to be close to the volume of the water alone, as the effect of adding the alcohol and contracting the volumes cancel out to some degree. But there would still be some leftover deviation. This contraction changes the volume and concentrations of the ions in solution, shifting equilibria slightly from what we calculated.
Dissolving vs dissociating
A common point of confusion is the difference between dissolution and dissociation. When something dissolves, it is being converted from a solid state to an aqueous state, meaning it gets separated and surrounded by water molecules (‘solvated’). This is a purely physical process: no ionisation necessarily occurs at this step! Dissociation, on the other hand, is the process where ionisation of the solvated species occurs. It is this process for which our equilibrium constants are defined. In all of the above calculation, we assumed that the benzyl alcohol dissolved completely in the water, meaning that no solid state was present at equilibrium. This essentially means that we assumed that benzyl alcohol is miscible in water, meaning it can be mixed in practically all proportions.
It is often said that magnesium hydroxide (
Solubility in organics can be inferred by the degree of hydrogen bonding between the solute and solvent, in comparison to solute-solute interactions. In our case of benzyl alcohol, the